

This is because audits by customers (2nd party audits) are becoming less common due to the rise of 1st party (internal) and 3rd party (external, for certification) audits.

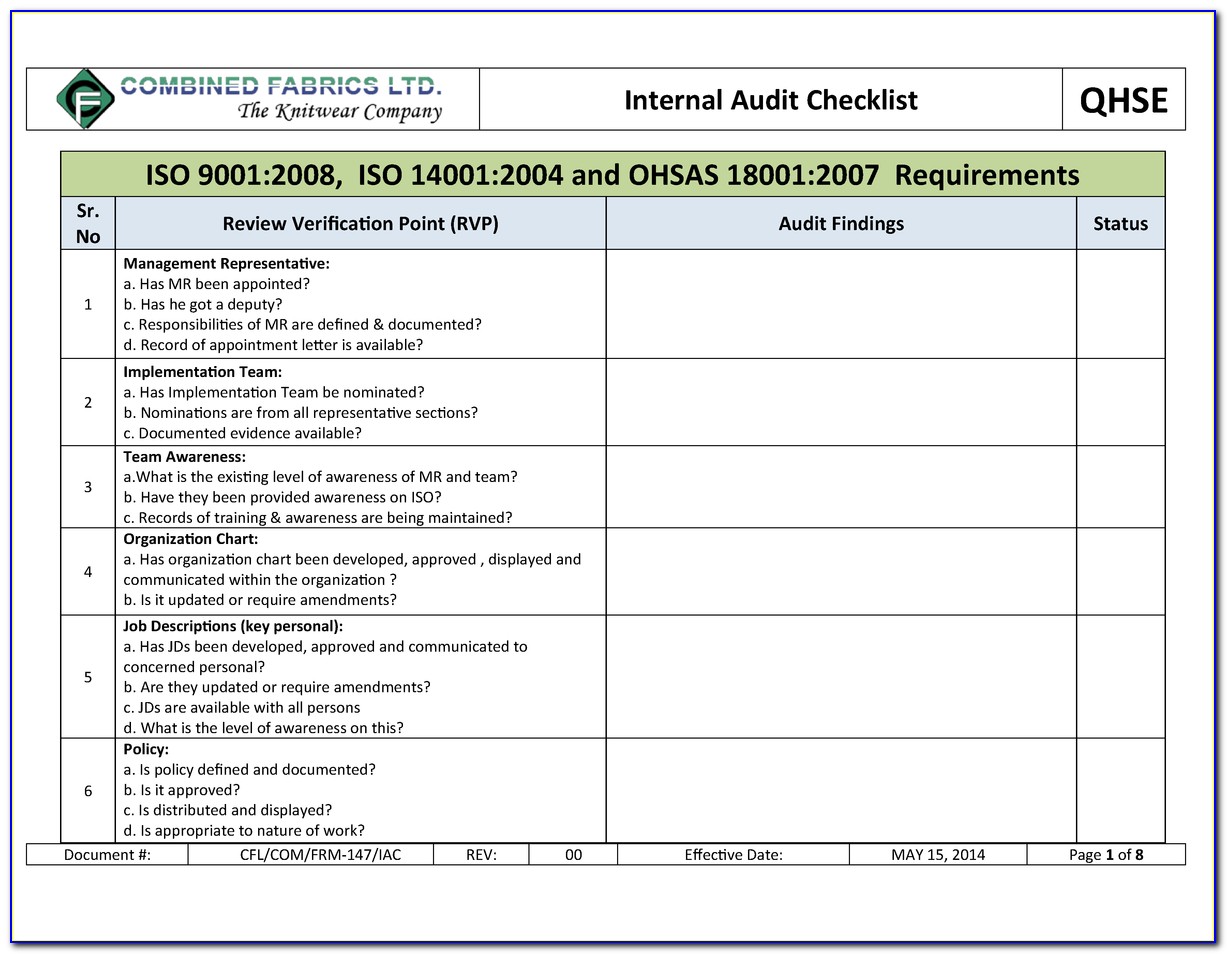
Iso 13485 internal audit checklist iso#
Increasingly, ISO 13485 is becoming necessary for medical devices companies to compete for customer attention. Design, development, production, distribution, servicing even supporting activities like maintenance and customer service.

Organizations using ISO 13485 can be involved in any stage of the medical devices life-cycle.
Requirements like those set out by ISO 13485 are strictly enforced throughout every stage of a medical device’s life-cycle, including stages after manufacturing like delivery, service, and maintenance. In the medical devices industry, quality management goes hand-in-hand with safety, and both are non-negotiables. The ISO 13485 framework also forms the basis for auditing these same organizations, for both internal and external audits. Perhaps the medical device industry’s most popular international standard for quality management, ISO 13485 provides a framework for manufacturers to implement the Medical Device Directives while simultaneously demonstrating a commitment to the quality and safety guidelines of medical devices.Īs of writing, the most recent version of the standard is ISO 13485:2016.īasically, ISO 13485 is like a quality management system for organizations involved in design, production, installation, and servicing of medical devices, with some other important requirements for good measure. Simply put, ISO 13485 is a set of requirements defined by The International Organization for Standardization, designed to be used by medical device manufacturers as a form of quality management system. In this article, I’ll break down the ISO 13485 standard, from a basic introduction to suggestions and resources for implementing it in your business or organization. Often, these kinds of requirements take the form of the ISO 13485 standard for medical device manufacturers. At some point soon, the current internal systems will not be able to hold back the deluge, and companies will be faced with a stark decision-consistently improve or perish” – Erik Myhrberg and Joseph Raciti, Practical Field Guide for ISO 13485 “On a global scale, we are all being asked to do more with less-and for less. That’s just the tip of the iceberg more severe penalties extend to include government bodies compelling you to dissolve your company, and ultimately the endangerment of the lives and well-being of individuals your organization is servicing. Failure to comply with several licencing regulations issued by the California Department of Insurance landed them a $7million fine. However, the struggle to turn a profit pales in comparison to some of the harsher consequences of failing to comply with certain regulatory requirements. In today’s business world, owners are constantly grappling with concerns and surmounting obstacles, the least of which is actually staying afloat financially in what can be an unforgiving economy.


 0 kommentar(er)
0 kommentar(er)
